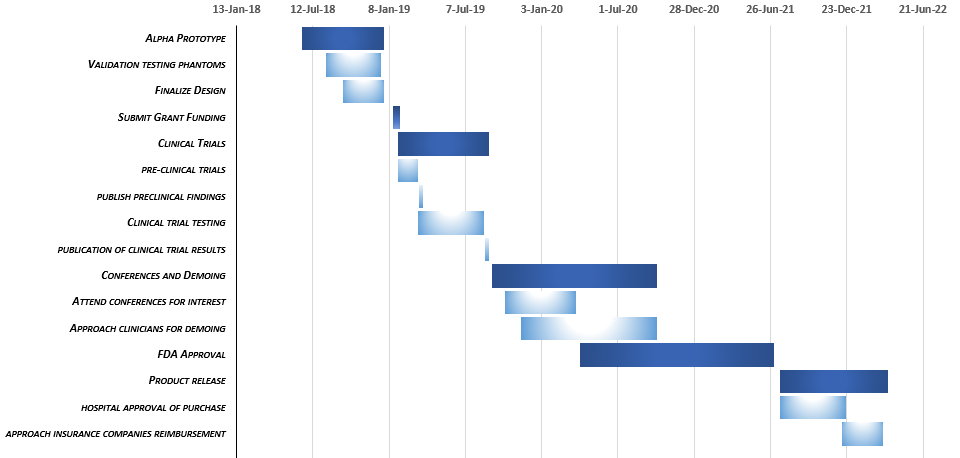
Business Strategy
Customers
Millions of patients every year are subject to the burden of pressure ulcers. In the United States alone, over 2.5 million individuals admitted to a hospital will develop a pressure induced ulcer. If these ulcers are detected in the later stages, the wound may become chronic and with eventual mortality. As a result, the customer base for this device is nurses and clinicians within hospitals that regularly maintain patients that are bedridden for extended periods of time.
Competitors
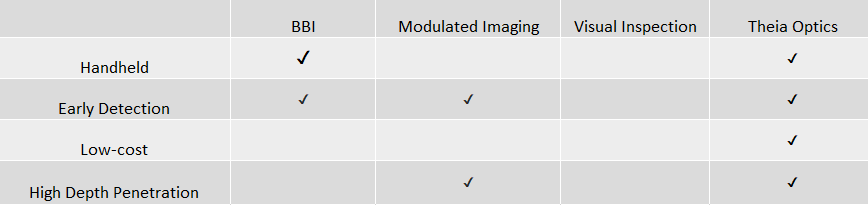
The current standard of practice for pressure ulcer identification remains archaic with the wound being examined visually after it has broken the surface of the skin. There are few devices on the market for pressure ulcer detection and said devices remain costly, difficult to maneuver, and bulky. In addition, other forms of utilized technology (such a laser speckle or laser doppler imaging) do not penetrate as deeply into the skin.
Business Plan